Linear DNA templates for rapid, versatile, and reliable mRNA production by
in vitro transcription
Linear dsDNA Up to 5.5 kb, with poly(A) tail
No downstream processing steps
Cell-free production: no risks of bioburden and endotoxins
Ships as fast as 10 business days
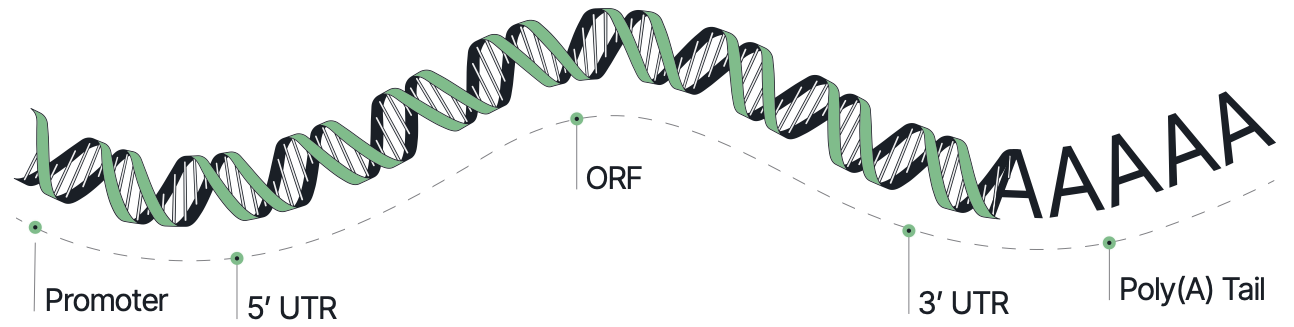
Built using Elegen’s patented cell-free DNA manufacturing technology, ENFINIA IVT Ready DNA templates offer a wide range of complexity without the pitfalls of plasmid cloning. Expand sequence diversity while reducing timelines and eliminating the risks of contamination and recombination.
Easy submission on
online portal
Complex sequences accepted – We can make what others can’t!
Selective amplification of perfect sequences
in vitro
No risk of bacterial contaminants

Proprietary method for poly(A) tail ligation & amplification
High poly(A) tail size consistency
Removal of impurities
✔️ Identity
✔️ Purity
✔️ Integrity
Reaction ready
Reliable
Stable poly(A) tail
Join the Elegen team as we explore a cell-free approach to generating high-complexity, linear IVT-ready DNA templates—built to accelerate and scale mRNA workflows. Hosted by DDN.
Don’t miss this deep dive into faster, more consistent mRNA production. Topics include:
• Key challenges in mRNA synthesis
• A robust, cell-free alternative to plasmid prep
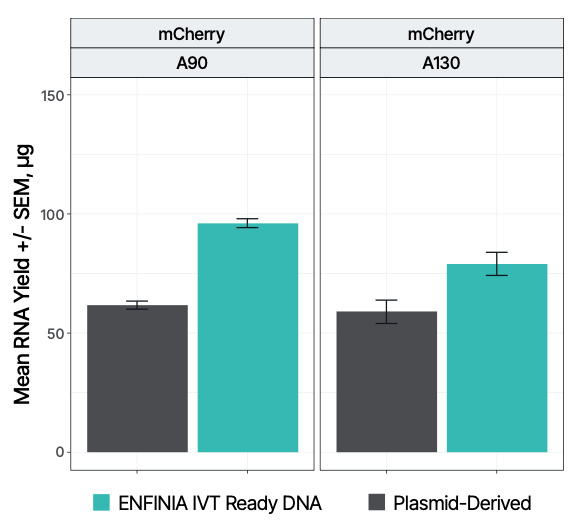
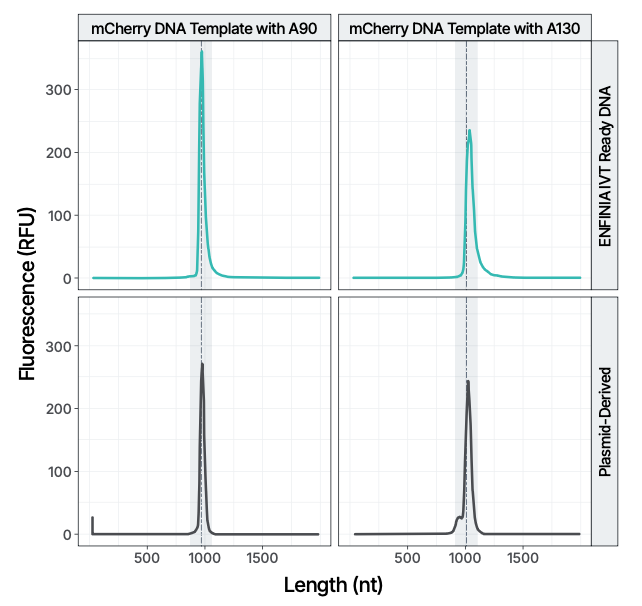
• Performance data comparing IVT-ready linear DNA vs. plasmid-based methods

Prophylactic Vaccines
Evaluate and select from more mRNA designs faster. Enable rapid mRNA screening, selection, and iteration—without the delays, variability, and endotoxin risks associated with plasmid-derived templates.
Protein Replacement Therapy
ENFINIA IVT Ready DNA enables fast and reliable mRNA production for therapeutic protein expression, without the complexity or cost of protein manufacturing or the risks of gene integration.
Personalized Cancer Vaccines
Rapidly test longer, polycistronic, and more complex neoantigen sequences without ever touching a cell.
 Cell Therapies & Genome Editing
Cell Therapies & Genome Editing
Leverage the large capacity of ENFINIA templates to encode genome editing modalities—such as Cas9 and base editors—for correcting genetic diseases and engineering the genome in vivo.
| Specification | Standard Complexity | High Complexity |
|---|---|---|
| Sequence Length (w/o poly(A) tail) | 1000-5,500 bp | 1000-5,500 bp |
| Overall GC Content | 25-65% | 25-76% |
| 100 bp GC Content | 22-75% | 12-83% |
| Local GC Variation | up to 60% | up to 70% |
| Repeats | up to 20 bp | up to 150 bp |
| Homopolymers* (not including poly(A) tail) | Up to 7 bases for G/C, Up to 8 bases for A/T | Up to 15 bases for G/C, Up to 30 bases for A/T |
*Elegen’s QC methods currently used for ENFINIA IVT Ready DNA may not reliably measure homopolymer sequences longer than 10 bp, except for the poly(A) tail. An accepted sequence containing a homopolymer that is longer than 10 bp may contain molecules where the homopolymer is shorter or longer than expected.
| Tail Type | Position 1 | Position 2 | Position 3 | Position 4 | Position 5 | Position 6 |
|---|---|---|---|---|---|---|
| Continuous Tails | A70 | A90 | A100 | A110 | A120 | A130 |
| Tail Type | Position 1 | Position 2 |
|---|---|---|
| Segmented Tails (two deoxyadenosine homopolymers separated by a 10 bp UGC Linker, 5'-GCATATGACT-3') | A30-Linker-A70 | A30-Linker-A90 |
| Sequence Length | Standard Complexity | High Complexity | Poly(A) Tail Fee | 10 µg | 50 µg |
|---|---|---|---|---|---|
| 1000 - 5500 bp | $0.07 /bp | $0.14 - $0.20 /bp | + $225 /seq | Included | + $1050 /seq |